Introduction to the Endocannabinoid System
Dustin Sulak, DO
Maine Integrative Healthcare
As you read this review of the scientific literature regarding the therapeutic effects of cannabis and cannabinoids, one thing will become quickly evident: cannabis has a profound influence on the human body. This one herb and its variety of therapeutic compounds seem to affect every aspect of our bodies and minds. How is this possible?
In my integrative medicine clinic in central Maine, we treat over a thousand patients with a huge diversity of diseases and symptoms. In one day I might see cancer, Crohn’s disease, epilepsy, chronic pain, multiple sclerosis, insomnia, Tourette’s syndrome and eczema, just to name a few. All of these conditions have different causes, different physiologic states, and vastly different symptoms. The patients are old and young. Some are undergoing conventional therapy. Others are on a decidedly alternative path. Yet despite their differences, almost all of my patients would agree on one point: cannabis helps their condition.
As a physician, I am naturally wary of any medicine that purports to cure-all. Panaceas, snake-oil remedies, and expensive fads often come and go, with big claims but little scientific or clinical evidence to support their efficacy. As I explore the therapeutic potential of cannabis, however, I find no lack of evidence. In fact, I find an explosion of scientific research on the therapeutic potential of cannabis, more evidence than one can find on some of the most widely used therapies of conventional medicine.
At the time of writing, a PubMed search for scientific journal articles published in the last 20 years containing the word “cannabis” revealed 7,704 results. Add the word “cannabinoid,” and the results increase to 15,899 articles. That’s an average of more than two scientific publications per day over the last 20 years! These numbers not only illustrate the present scientific interest and financial investment in understanding more about cannabis and its components, but they also emphasize the need for high quality reviews and summaries such as the document you are about to read.
How can one herb help so many different conditions? How can it provide both palliative and curative actions? How can it be so safe while offering such powerful effects? The search to answer these questions has led scientists to the discovery of a previously unknown physiologic system, a central component of the health and healing of every human and almost every animal: the endocannabinoid system.
What Is The Endocannabinoid System?
The endogenous cannabinoid system, named after the plant that led to its discovery, is perhaps the most important physiologic system involved in establishing and maintaining human health. Endocannabinoids and their receptors are found throughout the body: in the brain, organs, connective tissues, glands, and immune cells. In each tissue, the cannabinoid system performs different tasks, but the goal is always the same: homeostasis, the maintenance of a stable internal environment despite fluctuations in the external environment.
Cannabinoids promote homeostasis at every level of biological life, from the sub-cellular, to the organism, and perhaps to the community and beyond. Here’s one example: autophagy, a process in which a cell sequesters part of its contents to be self-digested and recycled, is mediated by the cannabinoid system. While this process keeps normal cells alive, allowing them to maintain a balance between the synthesis, degradation, and subsequent recycling of cellular products, it has a deadly effect on malignant tumor cells, causing them to consume themselves in a programmed cellular suicide. The death of cancer cells, of course, promotes homeostasis and survival at the level of the entire organism.
Endocannabinoids and cannabinoids are also found at the intersection of the body’s various systems, allowing communication and coordination between different cell types. At the site of an injury, for example, cannabinoids can be found decreasing the release of activators and sensitizers from the injured tissue, stabilizing the nerve cell to prevent excessive firing, and calming nearby immune cells to prevent release of pro-inflammatory substances. Three different mechanisms of action on three different cell types for a single purpose: minimize the pain and damage caused by the injury.
The endocannabinoid system, with its complex actions in our immune system, nervous system, and all of the body’s organs, is literally a bridge between body and mind. By understanding this system we begin to see a mechanism that explains how states of consciousness can promote health or disease.
In addition to regulating our internal and cellular homeostasis, cannabinoids influence a person’s relationship with the external environment. Socially, the administration of cannabinoids clearly alters human behavior, often promoting sharing, humor, and creativity. By mediating neurogenesis, neuronal plasticity, and learning, cannabinoids may directly influence a person’s open-mindedness and ability to move beyond limiting patterns of thought and behavior from past situations. Reformatting these old patterns is an essential part of health in our quickly changing environment.
What Are Cannabinoid Receptors?
Sea squirts, tiny nematodes, and all vertebrate species share the endocannabinoid system as an essential part of life and adaptation to environmental changes. By comparing the genetics of cannabinoid receptors in different species, scientists estimate that the endocannabinoid system evolved in primitive animals over 600 million years ago.
While it may seem we know a lot about cannabinoids, the estimated twenty thousand scientific articles have just begun to shed light on the subject. Large gaps likely exist in our current understanding, and the complexity of interactions between various cannabinoids, cell types, systems and individual organisms challenges scientists to think about physiology and health in new ways. The following brief overview summarizes what we do know.
Cannabinoid receptors are present throughout the body, embedded in cell membranes, and are believed to be more numerous than any other receptor system. When cannabinoid receptors are stimulated, a variety of physiologic processes ensue. Researchers have identified two cannabinoid receptors: CB1, predominantly present in the nervous system, connective tissues, gonads, glands, and organs; and CB2, predominantly found in the immune system and its associated structures. Many tissues contain both CB1 and CB2 receptors, each linked to a different action. Researchers speculate there may be a third cannabinoid receptor waiting to be discovered.
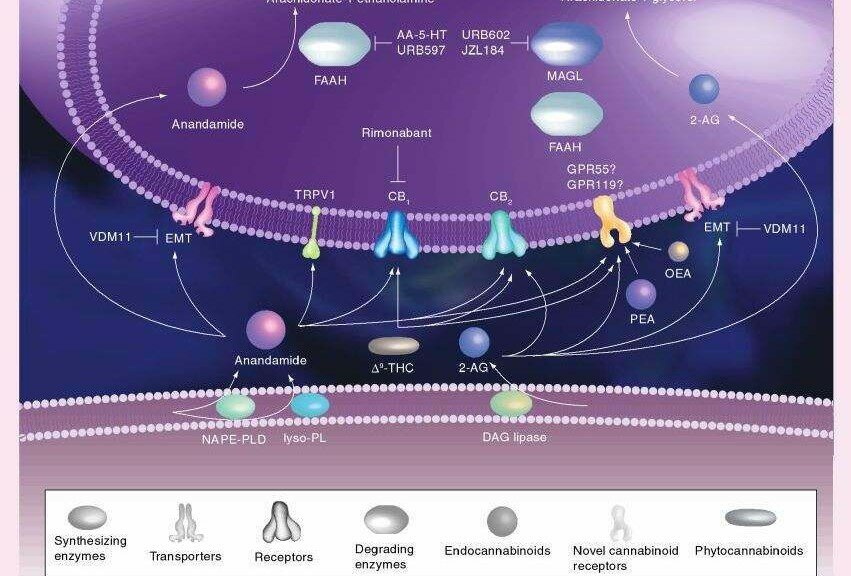
Endocannabinoids are the substances our bodies naturally make to stimulate these receptors. The two most well understood of these molecules are calledanandamide and 2-arachidonoylglycerol (2-AG). They are synthesized on-demand from cell membrane arachidonic acid derivatives, have a local effect and short half-life before being degraded by the enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL).
Phytocannabinoids are plant substances that stimulate cannabinoid receptors. Delta-9-tetrahydrocannabinol, or THC, is the most psychoactive and certainly the most famous of these substances, but other cannabinoids such as cannabidiol (CBD) and cannabinol (CBN) are gaining the interest of researchers due to a variety of healing properties. Most phytocannabinoids have been isolated from cannabis sativa, but other medical herbs, such as echinacea purpura, have been found to contain non-psychoactive cannabinoids as well.
Interestingly, the marijuana plant also uses THC and other cannabinoids to promote its own health and prevent disease. Cannabinoids have antioxidant properties that protect the leaves and flowering structures from ultraviolet radiation – cannabinoids neutralize the harmful free radicals generated by UV rays, protecting the cells. In humans, free radicals cause aging, cancer, and impaired healing. Antioxidants found in plants have long been promoted as natural supplements to prevent free radical harm.
Laboratories can also produce cannabinoids. Synthetic THC, marketed as dronabinol (Marinol), and nabilone (Cesamet), a THC analog, are both FDA approved drugs for the treatment of severe nausea and wasting syndrome. Some clinicians have found them helpful in the off-label treatment of chronic pain, migraine, and other serious conditions. Many other synthetic cannabinoids are used in animal research, and some have potency up to 600 times that of THC.
Cannabis, The Endocannabinoid System, And Good Health
As we continue to sort through the emerging science of cannabis and cannabinoids, one thing remains clear: a functional cannabinoid system is essential for health. From embryonic implantation on the wall of our mother’s uterus, to nursing and growth, to responding to injuries, endocannabinoids help us survive in a quickly changing and increasingly hostile environment. As I realized this, I began to wonder: can an individual enhance his/her cannabinoid system by taking supplemental cannabis? Beyond treating symptoms, beyond even curing disease, can cannabis help us prevent disease and promote health by stimulating an ancient system that is hard-wired into all of us?
I now believe the answer is yes. Research has shown that small doses of cannabinoids from marijuana can signal the body to make more endocannabinoids and build more cannabinoid receptors. This is why many first-time marijuana users don’t feel an effect, but by their second or third time using the herb they have built more cannabinoid receptors and are ready to respond. More receptors increase a person’s sensitivity to cannabinoids; smaller doses have larger effects, and the individual has an enhanced baseline of endocannabinoid activity. I believe that small, regular doses of marijuana might act as a tonic to our most central physiologic healing system.
Many physicians cringe at the thought of recommending a botanical substance, and are outright mortified by the idea of smoking a medicine. Our medical system is more comfortable with single, isolated substances that can be swallowed or injected. Unfortunately, this model significantly limits the therapeutic potential of cannabinoids.
Unlike synthetic derivatives, herbal marijuana may contain over one hundred different cannabinoids, including THC, which all work synergistically to produce better medical effects and less side effects than THC alone. While marijuana is safe and works well when smoked, many patients prefer to use a vaporizer or cannabis tincture. Scientific inquiry and patient testimonials both indicate that herbal marijuana has superior medical qualities to synthetic cannabinoids.
In 1902 Thomas Edison said, “There were never so many able, active minds at work on the problems of disease as now, and all their discoveries are tending toward the simple truth that you can’t improve on nature.” Cannabinoid research has proven this statement is still valid.
So, is it possible that medical marijuana could be the most useful remedy to treat the widest variety of human diseases and conditions, a component of preventative healthcare, and an adaptive support in our increasingly toxic, carcinogenic environment? Yes. This was well known to the indigenous medical systems of ancient India, China, and Tibet, and as you will find in this report, is becoming increasingly well known by Western science. Of course, we need more human-based research studying the effectiveness of marijuana, but the evidence base is already large and growing constantly, despite the DEA’s best efforts to discourage cannabis-related research.
Does your doctor understand the benefit of medical cannabis? Can he or she advise you in the proper indications, dosage, and route of administration? Likely not. Despite the two largest physician associations (American Medical Association and American College of Physicians) calling for more research, the Obama administration promising not to arrest patients protected under state medical cannabis laws, a 5,000 year history of safe therapeutic use, and a huge amount of published research, most doctors know little or nothing about medical cannabis.
This is changing, in part because the public is demanding it. People want safe, natural and inexpensive treatments that stimulate our bodies’ ability to self-heal and help our population improve its quality of life. Medical cannabis is one such solution. This summary is an excellent tool for spreading the knowledge and helping to educate patients and healthcare providers on the scientific evidence behind the medical use of cannabis and cannabinoids.
The multiple functions of the endocannabinoid system: a
focus on the regulation of food intake
Laboratory of Cardiovascular Investigation, Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro, Brazil
Diabetology & Metabolic Syndrome 2010, 2:5 doi:10.1186/1758-5996-2-5
The electronic version of this article is the complete one and can be found online at: http://www.dmsjournal.com/content/2/1/5
Abstract
Background
Cannabis sativa (also known as marijuana) has been cultivated by man for more than 5,000 years. However, there was a rise in its use in the 20th century for recreational, religious or spiritual, and medicinal purposes. The main psychoactive constituent of cannabis, whose structure was identified in the 1960′s, is Δ9-tetrahydrocannabinol. On the other hand, the discovery of cannabinoid receptors and their endogenous agonists took place only very recently. In fact, the first cannabinoid receptor (CB1) was cloned in 1990, followed 3 years later by the characterization of a second cannabinoid receptor (CB2). Since the 19th century, the use of cannabis has been reported to stimulate appetite and increase the consumption of sweet and tasty food, sometimes resulting in significant weight gain. The recent description of the endocannabinoid system, not only in the central nervous system but also in peripheral tissues, points to its involvement in the regulation of appetite, food intake and energy metabolism. Consequently, the pharmacological modulation of the over-activity of this system could be useful in the treatment of the metabolic syndrome.
Conclusions
The endocannabinoid system has important physiological functions not only in the central nervous system but also in peripheral tissues. The activation of central CB1 receptors, particularly in hypothalamic nuclei and in the limbic system, is involved in the regulation of feeding behavior, and especially in the control of the intake of palatable food. In the periphery, cannabinoid receptors are present in adipocytes, skeletal muscle, gastrointestinal tract and liver, modulating energy metabolism.
Introduction
Historical aspects
Cannabis sativa (marijuana or cannabis) has been cultivated by man since approximately 4,000 B.C [1,2]. At that time, the fibers obtained from the cannabis stems were mainly used to manufacture textiles and paper [1]. Moreover, from that time on, cannabis has also been known to have a variety of medicinal effects unrelated to its psychoactive properties, including effects on anorexia, emesis, pain, inflammation and neurodegenerative disorders [3]. Cannabis is the most widely used illicit drug in Western societies and also the one with the longest recorded history of human use. The popularity of marijuana as a recreational drug is due to its ability to alter sensory perception and cause elation and euphoria [2].
It has also been known since 300 B.C. that the recreational use of cannabis stimulates appetite, especially for sweet and palatable food [4,5]. Nevertheless, this phenomenon was seriously taken into consideration in biomedical research only in the last decade, after the description of the existence of an endogenous cannabinoid system [6,7], providing a physiological basis for the biological effects induced by cannabis and its derivatives.
Several chemical constituents of cannabis have already been identified, but its main psychoactive constituent is considered to be Δ9-tetrahydrocannabinol (Δ9-THC), whose structure was identified in the 1960′s [8]. Even though several naturally-occurring agonists of the endogenous cannabinoid system have been known since then, the discovery of cannabinoid receptors and their endogenous agonists took place only very recently. In fact, the first cannabinoid receptor (CB1) was cloned in 1990 [9], followed 3 years later by the characterization of a second cannabinoid receptor (CB2) [10].
The endocannabinoid system
Cannabinoid receptors belong to the G protein-coupled receptor superfamily and their activation modulates adenylate-cyclase, potassium and calcium channels and transcription factors such as mitogen-activated protein kinase [6,11]. The CB1 cannabinoid receptor is widely expressed in the central nervous system as well as in the periphery, while CB2 is mainly expressed in immune cells. In the central nervous system, CB1 is predominantly expressed presynaptically, modulating the release of neurotransmitters, including γ-aminobutyric acid (GABA), dopamine, noradrenaline, glutamate and serotonin [12].
The discovery of specific receptors mediating the actions of cannabis led to the search for endogenous ligands for cannabinoid receptors. The first endogenous cannabinoid, arachidonoyl ethanolamide, was identified in 1992 and was named anandamide, from the Sanskrit word ‘ananda’, meaning internal ecstasy[13,14]. Thus, both plant-derived (Δ9-THC) and endogenous (anandamide) agonists bind to the same cannabinoid receptors (Figure 1). Since the discovery of anandamide, other polyunsaturated fatty acid derivatives acting as functional agonists of cannabinoid receptors have been characterized and collectively termed endocannabinoids [15]. In contrast to classical neurotransmitters such as the catecholamines, endocannabinoids are not stored in the interior of synaptic vesicles because of the high lipophilicity of these ligands [6]. These findings led to the conclusion that the endocannabinoid system acts “on demand”, meaning that the endocannabinoids are synthesized and released upon physiological or pathological stimulation [6].
The endocannabinoid system and the regulation of food intake and energy metabolism
Since the 19th century the use of cannabis has been reported to stimulate appetite and to increase the consumption of sweet and tasty food, sometimes resulting in significant weight gain [4,16]. The recent description of the endocannabinoid system, not only in the central nervous system but also in peripheral tissues, points to its involvement in the regulation of appetite, food intake and energy metabolism [17-20]. Numerous experimental data have confirmed this hypothesis and will be briefly summarized below, while clinical results will be presented in the next topic of this article.
Endocannabinoid actions in the central nervous system
It has already been demonstrated that endocannabinoids initiate appetite by stimulation of CB1 receptors in hypothalamic areas involved in the control of food intake, such as the ventromedial hypothalamus (VMH) [21] (Figure 2). For instance, the injection of anandamide in the VMH of pre-satiated rats induces hyperphagia, which is prevented by previous hypothalamic administration of the selective CB1 cannabinoid antagonist rimonabant [22]. Using another experimental approach, Kirkham et al. [23] evaluated endocannabinoid levels in relation to the feeding behavior of rats in the hypothalamus and in the limbic forebrain, including the shell area of the nucleus accumbens, which is known to be linked to eating motivation. Endocannabinoid levels increased during fasting and declined during feeding, while no changes were detected in satiated rats. On the other hand, endocannabinoid levels in the cerebellum, which is not directly involved in the control of food intake, were unaffected by feeding behavior. Moreover, the injection of endocannabinoids into the nucleus accumbens induced feeding behavior, an effect that was also attenuated by rimonabant [23].
The experimental model of CB1 receptor knockout (CB1-/-) mice has also been used to clarify the involvement of the endocannabinoid system in the regulation of energy metabolism. An elegant study conducted by Ravinet-Trillou et al. compared CB1-/- knockout male mice with wild-type animals (CB1+/+) exposed either to a standard laboratory regimen or to a high-fat diet (HFD) [24]. When maintained on the standard diet, CB1-/- mice are lean and their body weight and adiposity are, respectively, 24 and 60% lower than that of CB1+/+ mice. CB1-/- mice submitted to the HFD do not develop obesity and do not display hyperphagia in contrast to CB1+/+ mice, and their feeding efficiency remains low. Furthermore, the insulin resistance that occurs in HFD-fed mice is not present in CB1-/- mice. These results led to the conclusion that CB1 receptors are involved not only in the development of diet-induced obesity but also in peripheral metabolic regulations [24]. These results were confirmed by Cota et al. [25], who showed that CB1-/- mice exhibit reduced spontaneous caloric intake and total fat mass, decreased body weight, and hypophagia, when compared with CB1+/+ littermates. Moreover, in young CB1-/- mice, the lean phenotype is predominantly caused by decreased caloric intake, whereas in adult CB1-/- mice, metabolic factors appear to contribute to the lean phenotype [25].
Another study carried out in mice submitted to HFD showed that a short-lasting reduction of food intake induced by a chronic oral treatment with rimonabant is dissociated from the long-lasting reduction of body weight and obesity. Moreover, rimonabant reverses the insulin resistance and lowered plasma leptin, insulin, and free fatty acid levels in this model of diet-induced obesity [26].
It is also noteworthy that Δ9-THC increases not only total food intake but also specifically increases the consumption of sweets, suggesting an interaction between the effect of the drug and the palatability of the food [27]. Another investigation confirmed this notion showing that different routes of Δ9-THC administration (oral, smoke inhalation or suppository) induced an effect on food selection [28]. Moreover, this selective action on food choice was confirmed in a number of animal studies, in which a potential role of cannabinoids in modulating the interaction of different pathways involved in the brain ‘reward’ system was hypothesized [29,30]. Thus, endocannabinoid signaling is involved orexigenically in both the homeostatic and the hedonistic control of food intake [31].
Endocannabinoid actions in the periphery
Adipocytes
A functional CB1 receptor has already been identified in the fat tissue of rodents [25]. The CB1 receptor is expressed both in epididymal fat pads extracted from mice and in primary adipocyte cell cultures and seems to be involved in the regulation of lipogenesis. Moreover, the receptor is expressed in fat tissue obtained from CB1+/+, but not CB1-/- knockout, mice. CB1 agonists dose-dependently increase lipoprotein lipase activity in adipocyte cell cultures, and this effect can be blocked by the preincubation with the CB1 selective antagonist rimonabant, thus confirming a specific cannabinoid mechanism of action [25].
Interestingly, the blockade of CB1 receptors, either in vivo or in adipocyte cell cultures, results in a significant increase of adiponectin production [32]. Adiponectin is secreted exclusively by adipose tissue, with plasma levels negatively correlated with obesity [33]. Moreover, adiponectin is the only adipocytokine with anti-inflammatory effects and with a protective role against atherosclerosis, in addition to its insulin sensitizer effect [34]. The modulation of the endocannabinoid system using a chronic oral treatment with rimonabant simultaneously reduces body weight and stimulates adiponectin mRNA expression in adipose tissue of obese Zucker rats [32]. In parallel, the hyperinsulinemia associated with this experimental model was reduced by rimonabant treatment [32]. Moreover, adiponectin expression is up-regulated by the blockade of CB1 receptors only in CB1+/+, but not CB1-/- knockout, mice [32].
Skeletal muscle
It has already been shown that the modulation of the endocannabinoid system regulates glucose uptake through the phosphatidylinositol-3-kinase pathway in skeletal muscle cells [35]. Accordingly, the blockade of peripheral CB1 receptors could contribute to the improvement of glycemia observed in clinical trials with rimonabant [36]. In this context, Liu et al. clearly demonstrated that a sub-acute treatment with rimonabant increases soleus muscle glucose uptake in leptin-deficient obese mice [37].
Liver
The presence of CB1 receptors in the mouse liver has been confirmed using multiple methodological approaches [38], indicating that the hepatocyte could be a peripheral molecular target of the endocannabinoid system. In fact, the activation of the CB1 receptor increases the expression of lipogenic genes in the liver, which is the main source of de novo fatty acid synthesis in the body [38].
Synthetic agonists of the CB1 receptor induce the expression of several key lipogenic enzymes in liver, an effect that is prevented by a pretreatment with CB1receptor antagonists [38]. Moreover, the basal rates of de novo fatty acid synthesis are markedly increased in CB1+/+ mice submitted to a high fat diet (HFD), compared with those of lean controls, but not in the CB1-/- knockout mice [38]. The pretreatment of CB1+/+ mice on HFD with a CB1 receptor antagonist reduces the rate of fatty acid synthesis [38]. In mice on HFD, the hepatic levels of the endocannabinoid anandamide were also greatly elevated. Finally, a marked deposition of lipid droplets was evident in wild-type, but not CB1-/- mice on HFD [38], suggesting the involvement of cannabinoid activation in liver steatosis.
Conclusions
The endocannabinoid system is present and has important physiological functions not only in the central nervous system but also in peripheral tissues. The activation of central CB1 receptors, particularly in hypothalamic nuclei and in the limbic system, is involved in the regulation of feeding behavior, and especially in the control of the intake of palatable food. It is important to mention that the activation of the endocannabinoid system, in addition to increasing appetite (total food intake), also influences the palatability and the preferential choice of tasty and sweet foods [5] (Figure 2). Thus, endocannabinoid antagonists could be utilized for the treatment of the type of obesity associated with specific eating disorders such as ‘sweet and snack-eating’ and compulsive eating episodes.
In the periphery, cannabinoid receptors are present in adipocytes, skeletal muscle, gastrointestinal tract and liver, modulating energy metabolism (Table 1).
Table 1. Central and peripheral sites of action of CB1 receptor antagonists and outcomes of the blockade of the endocannabinoid (EC) system.
In conclusion, the endocannabinoid system seems to play a key role in the development and maintenance of obesity (see previous reviews [5,39,40]). In obese patients, excessive food intake, especially of sweet and palatable food, could lead to the hyperactivity of the endocannabinoid system, resulting in several metabolic alterations typical of the metabolic syndrome (Figure 1). Thus, the pharmacological modulation of the endocannabinoid system turns out to be a unique target in the treatment of those that are overweight or obese [41].